In the ever-evolving landscape of pharmaceuticals, staying ahead of the curve is crucial for manufacturers. From production to packaging, the industry faces exciting challenges and opportunities. In this blog post, we’ll explore the future trends that will shape pharma production, manufacturing, and packaging. Buckle up as we delve into the world of innovation, sustainability, and compliance!
1. Advanced Manufacturing Techniques Continuous Manufacturing

Continuous manufacturing is revolutionizing the pharma industry. Unlike traditional batch processes, continuous manufacturing involves a seamless flow of raw materials, reactions, and product collection. Benefits include reduced production time, better quality control, and flexibility in scaling up or down.
3D Printing (Additive Manufacturing)

3D printing isn’t just for creating prototypes anymore. It’s making waves in drug formulation and personalized medicine. Imagine printing custom drug dosages based on individual patient needs. This technology promises efficiency, cost-effectiveness, and patient-centric solutions.
2. Sustainable Packaging Solutions Eco-Friendly Materials

Pharma companies are increasingly adopting sustainable packaging materials. Biodegradable plastics, recycled paper, and plant-based polymers reduce environmental impact. Consumers appreciate eco-friendly choices, and regulatory bodies encourage responsible packaging practices.
Smart Packaging
Smart packaging integrates technology to enhance safety and usability. Features like temperature monitoring, tamper-evident seals, and NFC-enabled labels provide real-time information to patients and healthcare providers. Smart packaging ensures product integrity and patient confidence.
3. Supply Chain Optimization
Blockchain Technology
Blockchain ensures transparency, traceability, and security throughout the supply chain. Pharma manufacturers can track raw materials, production processes, and distribution seamlessly. This technology minimizes counterfeiting risks and streamlines logistics.
Cold Chain Logistics
Biologics and vaccines require precise temperature control during transportation. Cold chain logistics use refrigerated containers, sensors, and data analytics to maintain product efficacy. As global distribution expands, optimizing cold chains becomes critical.
4. Regulatory Compliance
Serialization and Track-and-Trace
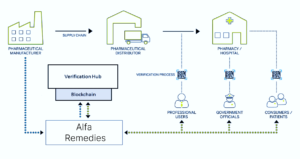
Regulatory agencies demand serialization and track-and-trace systems to prevent counterfeit drugs. Manufacturers must serialize each product unit and maintain a digital record of its journey. Compliance ensures patient safety and trust.
Data Integrity and Quality Management
Pharma companies must adhere to data integrity guidelines. Robust quality management systems ensure accurate documentation, process control, and adherence to Good Manufacturing Practices (GMP). Non-compliance can lead to severe penalties.
Conclusion
The future of pharma production, manufacturing, and packaging is dynamic and promising. Embrace innovation, sustainability, and compliance to thrive in this ever-evolving industry. Whether you’re a manufacturer, researcher, or healthcare provider, stay informed and adapt to the changing landscape.

