
In the pharmaceutical industry, the role of quality control cannot be overstated. It is a critical factor in ensuring the safety and efficacy of drugs. From raw material inspection to finished product testing, quality control plays a pivotal role at every stage of pharma manufacturing.
The Importance of Quality Control

Quality control in pharma manufacturing is not just about meeting regulatory requirements. It’s about ensuring that every drug that reaches the market is safe for consumption and effective in treating the condition it’s meant for. This is particularly important in an industry where the end products are consumed by patients, often in critical conditions. The slightest deviation in quality can have serious implications on a patient’s health. Therefore, stringent quality control measures are not just a regulatory requirement, but a moral obligation for every pharmaceutical manufacturer.
Quality Control Processes in Pharma Manufacturing
Quality control in pharma manufacturing involves a series of processes. Each of these processes is designed to ensure that the final product meets the highest standards of quality and safety. Here’s a closer look at these processes:
1. Raw Material Inspection
Every drug starts with raw materials. These could be active pharmaceutical ingredients (APIs), excipients, or other substances used in the formulation of the drug. Quality control ensures that these materials meet the required standards. This involves checking the purity, strength, and quality of the materials. Any contamination or deviation from the specified standards can affect the quality of the final product.
2. In-Process Quality Control

During the manufacturing process, quality control measures are put in place to ensure that each batch of drugs is produced consistently. This involves monitoring the manufacturing process and making adjustments as necessary to maintain the desired quality. In-process quality control helps in early detection of any deviations, allowing for timely corrective actions.
3. Finished Product Testing
Once the drugs are manufactured, they undergo rigorous testing to ensure their safety and efficacy. This includes physical and chemical testing, as well as biological testing where necessary. Only those batches that pass all the tests are approved for distribution.
The Future of Quality Control in Pharma Manufacturing
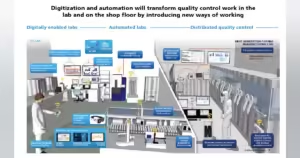
With advancements in technology, the future of quality control in pharma manufacturing looks promising. From AI-powered inspection systems to real-time quality control, the industry is set to become more efficient and reliable. These advancements will not only improve the quality of drugs but also speed up the manufacturing process, thereby reducing costs and increasing accessibility of drugs.
Conclusion
Quality control is the backbone of pharma manufacturing. It not only ensures the safety and efficacy of drugs but also builds trust with consumers. As the pharmaceutical industry continues to evolve, quality control will remain a key focus area, driving the industry towards higher standards of quality and safety.

